71 LectureDouble Replacement ReactionsPrecipitation Reaction insoluble productGas Forming Reaction bubblesNeutralization Reaction water formedNo React. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
Solved Understanding These Concepts Just Like We Did In Chegg Com
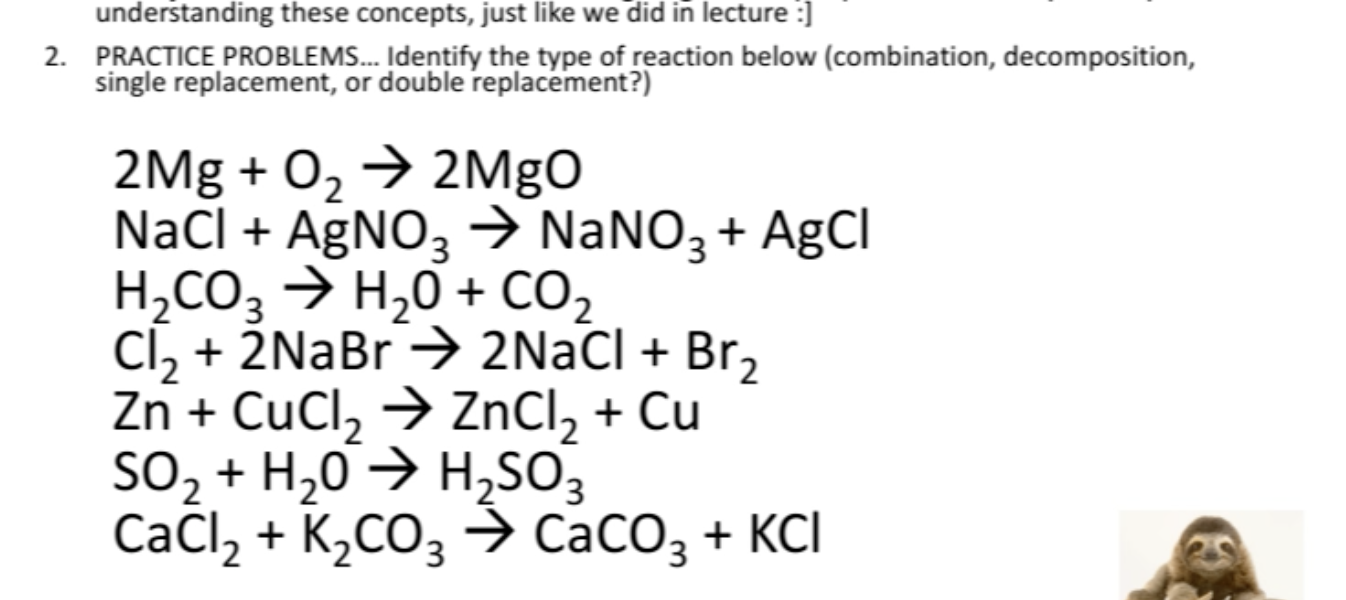
1 CaOH2 H3PO4--- Ca3PO42 H2O.
. In Chemistry University of Connecticut. Nitric acid lithium sulfide. Back to Double Replacement.
Predict the products for and then balance each of the following chemical reactions. Aluminum iodide mercuryII chloride Æ 2. 4 CoOH3 HNO3--- CoNO33 H2O.
Shes very detail oriented and goes over every step carefully. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. Predicting products of chemical reactions - practice problems Directions.
Learn vocabulary terms and more with flashcards games and other study tools. 2Al 3I2 synthesis 3. If youre seeing this message it means were having trouble loading external resources on our website.
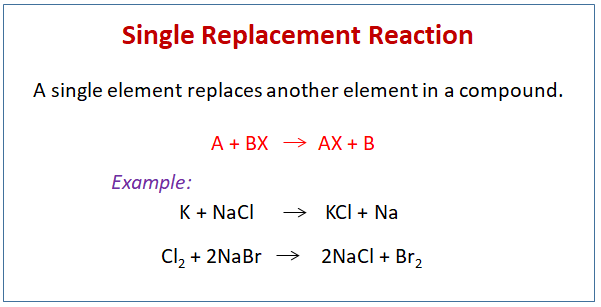
The chemical bonds between the reactants may be either covalent or ionic. 2 K2CO3 BaCl2--- KCl BaCO3. SiI4 Mg single replacement 2.
In Physical Chemistry UC Berkeley BS. Iron II sulfate sodium phosphate. No DR Reaction A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
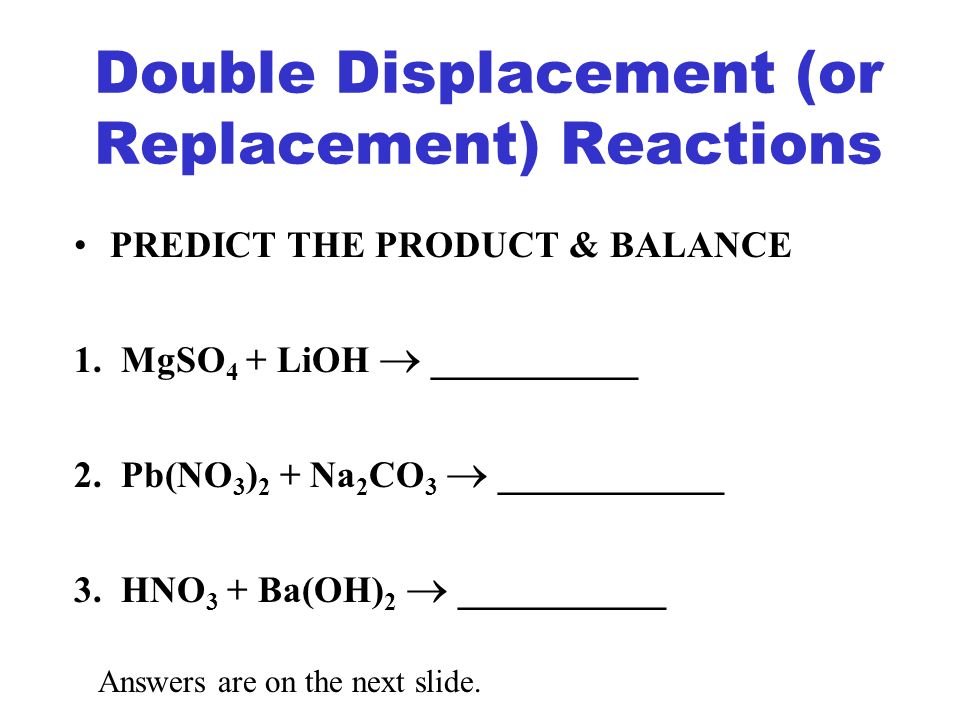
A double replacement reaction can be defined as a reaction in which one set of ions of the same charge are exchanged and two new products are formed. Double-Replacement Reactions In these reactions all you do is look at the names of the reactants and switch partners. Usually a double displacement reaction results in precipitate formation.
3 Cd3PO42 NH42S --- CdS NH43PO4. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. Note that none of the example problems above are balanced.
Select two compounds above and this calculator will predict whether or not the reaction will occur in water. DOUBLE REPLACEMENT PRACTICE REACTIONS For each reaction predict the products and balance the equation. However there is one type of double-replacement reaction that we can predict.
Pleasecompletethefollowingdoublereplacementreactions Youshouldbeattemptingtowriteallequationsasnetionicequations 1. Lets practice problems involving displacement and double displacement reactions. Predict the products and write balanced equations for the following double displacement reactions.
AgNO 3 NaCl --- Silver nitrate sodium chloride silver chloride sodium nitrate AgNO 3 NaCl --- AgCl NaNO 3 1 CaOH 2 H 3 PO 4--- 2 K 2 CO 3 BaCl 2--- 3. Neutralization precipitation and gas formation are types of double. Just be sure that the new pairs come out with the positive ion named first and paired with a negative ion.
Classify as a combination decomposition single replacement double replacement or combustion reaction. It covers three types. Start studying Synthesis Decomposition Single Replacement Double Replacement Practice.
CuCl2 KOH double replacement 4. Na 1 aq Br 1- aq H 1 aq Cl 1- aq. C4H10 O2 combustion 7.
Nickel II chloride potassium bromide. Your teacher may require this but the ChemTeam will only provide some of the following answers balanced. It must be noted that the results are not 100.
Write correct formulas for the products in these double replacement reactions. Need help with chemistry. Lithium carbonate magnesium bromide.
She has an amazing talent for explaining the most difficult concepts in very easy-to-understand terms. Double Replacement Reactions - Problem 1. It can be illustrated as follows.
This new compound falls out of solution as a solid precipitate. Ammonium permanganate lithium hydroxide. Write correct formulas for the products in these double replacement reactions.
If youre behind a web filter please make sure that the. Mg HCl single replacement 6. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
The overall pattern of a double replacement reaction looks like this. A precipitation reaction occurs when two ionic compounds are dissolved in water and form a new ionic compound that does not dissolve. State the reaction in chemical formulas and in symbols.
This chemistry video tutorial explains how to identify the products of a double replacement reaction from a sentence or word problem. A double displacement reaction is also called a double replacement. The rest are up to you.
1 CaOH 2 H 3 PO 4--- 2 K 2 CO 3 BaCl 2---. This is simply based on the solubility chart of inorganic compounds.
Double Displacement Or Replacement Reactions Ppt Download

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Single Displacement Reactions Examples Solutions Videos

Chemistry Practice Problems Double Displacement Reactions Youtube

Double Replacement Rxn Worksheet

Double Replacement Reaction Definition And Examples

Single Replacement Reactions And Net Ionic Equations Youtube

Double Replacement Reaction Practice Problems Examples Youtube
0 comments
Post a Comment